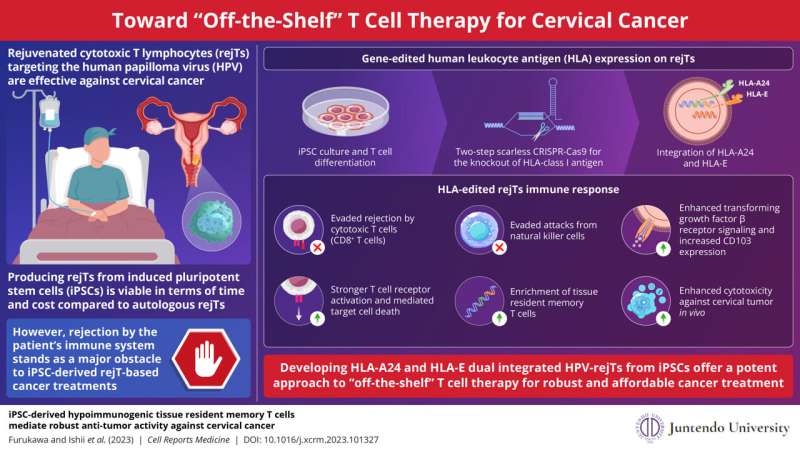
A team including University of Wyoming anthropologists works at the site of a circular plaza that was built around 4,750 years ago in the Cajamarca Basin of northern Peru.
Two University of Wyoming anthropology professors have discovered one of the earliest circular plazas in Andean South America, showcasing monumental megalithic architecture, which refers to construction that uses large stones placed upright with no mortar.
Located at the Callacpuma archaeological site in the Cajamarca Basin of northern Peru, the plaza is built with large, vertically placed megalithic stones—a construction method previously unseen in the Andes. Associate Professor Jason Toohey, project lead, and Professor Melissa Murphy have been researching this topic since the project’s inception in 2015. Excavations took place in the plaza starting in 2018.
Their paper, which reports new data on this earliest known megalithic circular plaza in the northern Andes, is titled “A Monumental Stone Plaza at 4750 BP in the Cajamarca Valley of Peru” and has been published in Science Advances.
Radiocarbon dating places its initial construction around 4,750 years ago during the Late Preceramic Period, making it one of the earliest instances of such architecture in the Americas.
To better understand this timeline, the team carefully excavated within the plaza, uncovering artifacts related to life in the past and collecting charcoal samples for dating. All material remains then were cleaned, processed and analyzed in the laboratory.
“This structure was built approximately 100 years before the Great Pyramids of Egypt and around the same time as Stonehenge,” Toohey says.
These dates signify that the circular plaza at Callacpuma is the earliest known example of monumental and megalithic architecture in the Cajamarca Valley—and one of the earliest examples in ancient Peru.
“It was probably a gathering place and ceremonial location for some of the earliest people living in this part of the Cajamarca Valley,” Toohey adds. “These people were living a primarily hunting-and-gathering lifestyle and probably had only recently begun growing crops and domesticating animals.”
The plaza is formed by two concentric walls and measures about 60 feet in diameter.
The project is led by Toohey and Patricia Chirinos Ogata from the University of California-Santa Barbara. The team also includes Murphy, as well as undergraduate and graduate students from Peru and the U.S.
Toohey is an anthropological archaeologist who is dedicated to taking a holistic and multidisciplinary approach to the field. He has conducted fieldwork in the Peruvian Andes since 2003. The department head for anthropology at UW, Murphy is a biological anthropologist specializing in bioarchaeology and committed to multidisciplinary approaches within anthropology.
“As part of our community outreach, we collaborate and work with the residents of the towns on and adjacent to the site of Callacpuma about our findings and their importance,” Toohey says. “We highlight the importance of cultural heritage, and working together, we can continue the scientific investigations and help to preserve the site.”